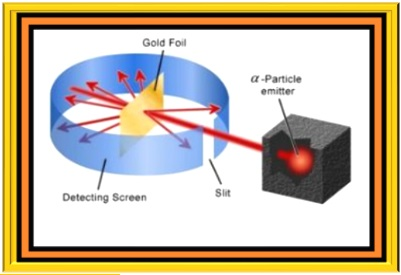
Rutherford designed an experiment to find out how electrons are arranged in an atom. In this experiment, fast moving alpha `color{red}(α)`-particles were made to fall on a thin gold foil.
`color{green}(•)` He selected a gold foil because he wanted as thin a layer as possible. This gold foil was about `color{red}(1000)` atoms thick.
`color{green}(•)` `color{red}(α)`-particles are doubly-charged helium ions. Since they have a mass of `color{red}(4 u)`, the fast-moving `color{red}(α)`-particles have a considerable amount of energy.
`color{green}(•)` It was expected that `color{red}(α)`-particles would be deflected by the sub-atomic particles in the gold atoms. Since the `color{red}(α)`-particles were much heavier than the protons, he did not expect to see large deflections.
`color{green}("𝐓𝐡𝐞 𝐟𝐨𝐥𝐥𝐨𝐰𝐢𝐧𝐠 𝐨𝐛𝐬𝐞𝐫𝐯𝐚𝐭𝐢𝐨𝐧𝐬 𝐰𝐞𝐫𝐞 𝐦𝐚𝐝𝐞:")`
(i) Most of the fast moving `color{red}(α)`-particles passed straight through the gold foil.
(ii) Some of the `color{red}(α)`-particles were deflected by the foil by small angles.
(iii) Surprisingly one out of every `color{red}(12000)` particles appeared to rebound.
`color{green}("𝐂𝐎𝐍𝐂𝐋𝐔𝐒𝐈𝐎𝐍 𝐎𝐅 𝐑𝐔𝐓𝐇𝐄𝐑𝐅𝐎𝐑𝐃’𝐒 𝐌𝐎𝐃𝐄𝐋 𝐎𝐅 𝐀𝐍 𝐀𝐓𝐎𝐌;")`
(i) Most of the space inside the atom is empty because most of the `color{red}(α)`-particles passed through the gold foil without getting deflected.
(ii) Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
(iii) A very small fraction of `color{red}(α)`-particles were deflected by `color{red}(180^0)`,indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.


From the data he also calculated that the radius of the nucleus is about `color{red}(10^5)` times less than the radius of the atom..
Results of Rutherford model of an atom:
(i) There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
(ii) The electrons revolve around the nucleus in well-defined orbits.
(iii) The size of the nucleus is very small as compared to the size of the atom.
`color{green}("𝐃𝐫𝐚𝐰𝐛𝐚𝐜𝐤𝐬 𝐨𝐟 𝐑𝐮𝐭𝐡𝐞𝐫𝐟𝐨𝐫𝐝’𝐬 𝐦𝐨𝐝𝐞𝐥 𝐨𝐟 𝐭𝐡𝐞 𝐚𝐭𝐨𝐦:")`
Any particle in a circular orbit would undergo acceleration. During acceleration, charged particles would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable and hence matter would not exist in the form that we know. We know that atoms are quite stable.

Rutherford designed an experiment to find out how electrons are arranged in an atom. In this experiment, fast moving alpha `color{red}(α)`-particles were made to fall on a thin gold foil.
`color{green}(•)` He selected a gold foil because he wanted as thin a layer as possible. This gold foil was about `color{red}(1000)` atoms thick.
`color{green}(•)` `color{red}(α)`-particles are doubly-charged helium ions. Since they have a mass of `color{red}(4 u)`, the fast-moving `color{red}(α)`-particles have a considerable amount of energy.
`color{green}(•)` It was expected that `color{red}(α)`-particles would be deflected by the sub-atomic particles in the gold atoms. Since the `color{red}(α)`-particles were much heavier than the protons, he did not expect to see large deflections.
`color{green}("𝐓𝐡𝐞 𝐟𝐨𝐥𝐥𝐨𝐰𝐢𝐧𝐠 𝐨𝐛𝐬𝐞𝐫𝐯𝐚𝐭𝐢𝐨𝐧𝐬 𝐰𝐞𝐫𝐞 𝐦𝐚𝐝𝐞:")`
(i) Most of the fast moving `color{red}(α)`-particles passed straight through the gold foil.
(ii) Some of the `color{red}(α)`-particles were deflected by the foil by small angles.
(iii) Surprisingly one out of every `color{red}(12000)` particles appeared to rebound.
`color{green}("𝐂𝐎𝐍𝐂𝐋𝐔𝐒𝐈𝐎𝐍 𝐎𝐅 𝐑𝐔𝐓𝐇𝐄𝐑𝐅𝐎𝐑𝐃’𝐒 𝐌𝐎𝐃𝐄𝐋 𝐎𝐅 𝐀𝐍 𝐀𝐓𝐎𝐌;")`
(i) Most of the space inside the atom is empty because most of the `color{red}(α)`-particles passed through the gold foil without getting deflected.
(ii) Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space.
(iii) A very small fraction of `color{red}(α)`-particles were deflected by `color{red}(180^0)`,indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom.


From the data he also calculated that the radius of the nucleus is about `color{red}(10^5)` times less than the radius of the atom..
Results of Rutherford model of an atom:
(i) There is a positively charged centre in an atom called the nucleus. Nearly all the mass of an atom resides in the nucleus.
(ii) The electrons revolve around the nucleus in well-defined orbits.
(iii) The size of the nucleus is very small as compared to the size of the atom.
`color{green}("𝐃𝐫𝐚𝐰𝐛𝐚𝐜𝐤𝐬 𝐨𝐟 𝐑𝐮𝐭𝐡𝐞𝐫𝐟𝐨𝐫𝐝’𝐬 𝐦𝐨𝐝𝐞𝐥 𝐨𝐟 𝐭𝐡𝐞 𝐚𝐭𝐨𝐦:")`
Any particle in a circular orbit would undergo acceleration. During acceleration, charged particles would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable and hence matter would not exist in the form that we know. We know that atoms are quite stable.